Environmental and biological controls on soil organic matter formation
Where does soil organic matter (SOM) come from?
Our fundamental view of SOM dynamics has transformed in the past couple decades; we now know that soil microorganisms are the key regulators of SOM formation. While general mechanisms controlling SOM stabilization and destabilization are somewhat known, exact mechanisms and optimal conditions for building SOM vary depending on local conditions. Control of SOM formation and degradation is regulated by processes related to microbial activity and growth rates and by physical protection of SOM. Microorganisms contribute to SOM formation by breaking down organic matter into small, low molecular weight compounds that are highly oxidized and more easily subject to physiochemical protection and adsorbed to mineral surfaces. Evidence also suggests that microbial necromass is a significant source of SOM and the potential for this necromass to form long-lived SOM varies with microbial community composition. Given that microbially derived compounds account for a substantial proportion of stabilized SOM and microbial necromass alone may account for 40-80% of SOM, the structure of microbial communities and their activity, particularly growth efficiencies, are likely very important determinants of SOM formation and stabilization. Under this emerging view, it is critical to understand differences in microbial C preferences, physiological traits (e.g. microbial growth and biomass turnover rates and C use efficiency) and interactions at the mineral-microbe interface that influence the chemical structure and nature of the material destined to become SOM.
Mechanisms for increased soil C storage with increasing rotational diversity in agroecosystems
Author: Lisa Tiemann
Generally, there are positive relationships between plant species diversity and net primary production and other key ecosystem functions. However, the effects of aboveground diversity on soil microbial communities and ecosystem processes they mediate, such as soil C sequestration, remain unclear. In this study, we used an 11-y cropping diversity study where increases in diversity have increased crop yields. At the experimental site, temporal diversity is altered using combinations of annual crop rotations, while spatial diversity is altered using cover crop species. We used five treatments ranging in diversity from one to five species consisting of continuous corn with no cover crop or one cover crop and corn-soy-wheat rotations with no cover, one cover or two cover crop species. We collected soils from four replicate plots of each treatment and measured the distribution of mega- (>2 mm), macro- (0.25–2 mm), and micro- (0.053-0.25 mm) aggregates. Within each aggregate size class, we also measured total soil C and N, permanganate oxidizable C (POXC), extracellular enzyme activities (EEA), and microbial community structure with phospholipid fatty acid (PLFA) analysis. We use these data to address the impacts of both rotational and cover crop diversity on soil physical structure, associated microbial community structure and activity and soil C storage.
As spatial diversity increased, we found concurrent increases in mega-aggregate abundance as well as increasing soil C in the mega- and micro-aggregates but not macro-aggregates. The proportion of total soil C in each aggregate size class that is relatively labile (POXC) was highest in the micro-aggregates, as was enzyme activity associated with labile C acquisition across all levels of diversity. Enzyme activity associated with more recalcitrant forms of soil C was highest in the mega-aggregate class, also across all diversity levels; however, the ratio of labile to recalcitrant EEA increased with increasing diversity in the mega- and micro-aggregates. In addition, soil N increased with diversity such that microbial C:N EEA simultaneously decreased in mega-aggregates. We also found that cropping diversity has created distinctive soil microbial communities, highlighted by variation in the abundance of gram positive bacteria and Actinomycetes. Further research will help us determine how these changes in community structure with increasing diversity are related to concomitant changes in aggregation and enzyme activities. We suggest that the additional organic matter inputs from cover crops in the high diversity treatments have increased aggregation processes and C pools. While microbial activity has also increased in association with this increased C availability, the activity of recalcitrant and N-acquiring enzymes has declined, suggesting an overall decrease in SOM mineralization with possible increased SOM stabilization. The addition of crop species in rotation (temporal diversity) had minimal influence on any of the measured parameters. We thus conclude that spatial diversity is a more important driver of soil structure and microbial activity, likely due to the high quality organic matter inputs derived from the leguminous cover crops; however, spatial diversity alone did not lead to the same level of C storage potential as mixtures of temporal and spatial diversity.
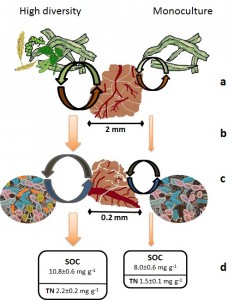
Trajectory of aggregate and SOM formation and stabilization under a high diversity rotation (e.g. SWC2) versus monoculture crop (e.g. Cm). (a) Greater quantity and quality of residues entering soils in high diversity rotations enhances microbial activity with positive impacts on rates and extent of mega-aggregate formation and stabilization. This also leads to (b) enhanced microbial activity in high diversity micro-aggregates and concomitant increases in microbial by-products that accelerates micro-aggregate formation, resulting in (c) increasing stocks of stable SOC and TN.
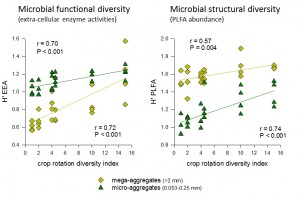
Crop rotation diversity index versus (a) microbial enzyme activity diversity and (b) PLFA diversity in relation in mega- or micro-aggregates. The calculated Shannon-Weiner diversity (H) is relevant for between rotation comparisons at this site, but is not a ‘true’ measure of diversity because maximum richness was determined a priori.

